SAFETY OF MEDICAL ELECTRICAL EQUIPMENT
IEC 60601-1 is the safety standard for medical electrical equipment and covers all types of equipment within the industry, from small to large scale equipment. For example:
- Infusion pumps
- Gamma Equipment
- Handheld wellness device
- Laser Therapeutic device
- Ultrasound device
- Blood pressure device
- And much more!
It covers any active device that is powered by electricity, such as a battery-operated device, a wall powered device or a nuclear device.
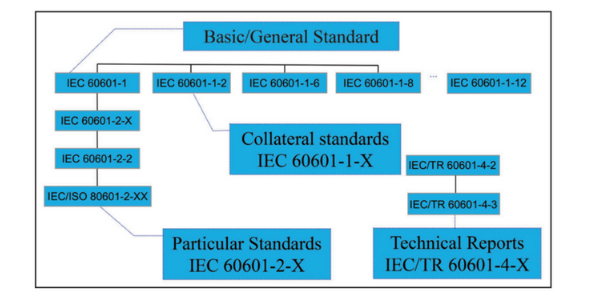
The Standard follows the same general requirements that IEC 60950-1 contains, however, due to the nature of the product and the fact that the product is going to be used medically to treat or cure patients (in contact with a patient), there is an increased level of protection and safety tests that are found within the standard, such as:
- AC Hipot – Designed to verify the integrity of insulation using AC Voltage.
- SC Hipot – Designed to verify the integrity of insulation using DC Voltage.
- Ground Bond – Designed to test the integrity of the ground circuit on the device.
- Leakage Current – That can include, touch current, patient current, auxiliary current, mains and apply part leakage.
IEC 60601-1 has had a few revisions since its existence in 1988. The 2nd edition of IEC 60601-1 focused on safety within a 6-foot radius from a patient also known as “patient vicinity”, the three categories of increasing severity were defined:
- F-Type – Applied Part which is electrically isolated from Earth and other parts of the medical equipment. i.e. floating. F-type Applied Parts are either type BF or type CF Applied Parts.
- B-Type – Applied Part complying with specified requirements for protection against electric shock. Type B Applied Parts are those parts, which are usually Earth referenced. Type B are those parts not suitable for direct cardiac application, such as MRI scanners, X-ray machines and LED operating lighting.
- BF-Type – F-Type Applied Part comply with a higher degree of protection against electric shock than type B Applied Parts. Type BF Applied Parts are those parts not suitable for the direct cardiac application, such as ultrasound equipment, thermometers, and blood pressures devices.
- CF-Type – Applied Part complying with the highest degree of protection against electric shock. Type CF Applied Parts are those parts suitable for the direct cardiac application, such as defibrillators and dialysis machines.
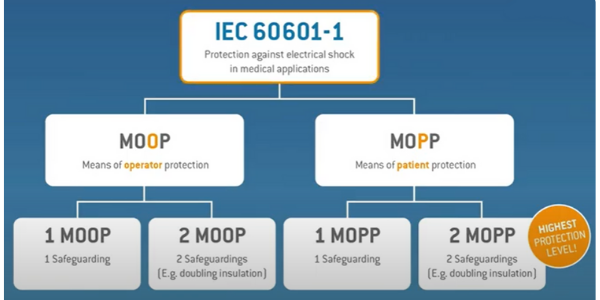
A 3rd edition was introduced in 2005, this was introduced to look at the different “means of protection” (MOP) for both patients and operators.
As you can see from the diagram, the type of MOP protection that a customer would need depends on the two factors within the diagram, MOOP (Means of operator protection) and MOPP (Means of patient protection). Medical power supplies for type BF and CF medical devices are designed to provide 2 x MOPP of input to output isolation.
This edition of separating “Operator” and “Patient” was necessary because whilst the health care professional (operator) has access to the control panel, the patient might be connected to the device receiving treatment, meaning the hazards differ from operator to patient. Electric currents that run through the body are extremely dangerous and often result in death. For a healthy person, currents as low as 40mA could be fatal. For a person who is anaesthetised or weakened by an illness, this threshold is likely to be much lower.
